Core data: global market size of high-value medical consumables industry;
By 2024, the global market size of the high-value medical consumables industry will be close to $180 billion
High-value medical consumables generally refer to expendable medical devices that are critical to safety, must be strictly controlled for production and use, are limited to the use of certain specialties and have relatively high prices. In 2023, the global market size of high-value medical consumables exceeded 165 billion US dollars, and the five-year industry compound growth rate was 5.69%. According to preliminary estimates, the global market size of high-value medical consumables industry will be close to $180 billion in 2024.

Market segmen
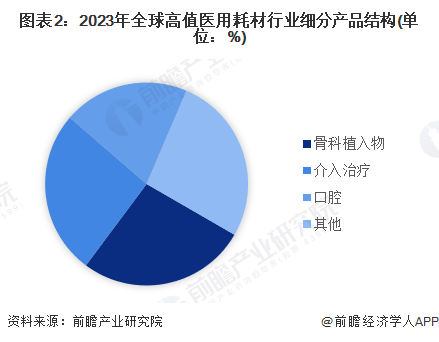
According to the "Blue Book of Medical Devices" jointly issued by China Drug Regulatory and Administration Research Society and Social Sciences Academic Press, high-value medical consumables are divided into vascular intervention, orthopaedic implant, blood purification, oral, surgical, ophthalmic, non-vascular intervention and neurosurgery. According to the data, in the global high-value medical consumables market segment in 2023, the orthopedic implant market share accounted for about 27%.
Industrial competition
Global high-value medical consumables are mainly European and American companies such as Medtronic, Johnson & Johnson, Stryker, etc., occupy the main market share, and these companies dominate with technological innovation, brand influence and a wide range of product lines. With the advancement of medical technology, enterprises have increased investment in research and development, and launched more efficient and accurate medical products. At the same time, the diversification of market demand has promoted competition in segments such as cardiology, orthopedics, neurosurgery and other fields. Emerging companies are gradually challenging traditional giants through innovation and cost control, and the global market is showing a rapid growth trend as countries attach importance to healthcare.

|
Last:Reprint:Reform of drug and medical device supervision
Next:Reprint:Analysis of import and export of medical imaging equipment industry in China in 2024 |
Return |